
NH3 (ammonia) Lewis dot structure YouTube
The Lewis structure of nitrogen and hydrogen atom shows a total of eight valence electrons participating in a bond formation, to produce a single tetra-atomic NH3 molecule. Here, we need to study how the Lewis structure of the NH3 molecule is drawn: Search the total number of valence electrons: It is eight to form a single NH3 molecule.

Is NH3 Polar or Nonpolar?Is Ammonia a Polar or Nonpolar Molecule?
= 8 valence Ammonia or NH3 has a total of 8 valence electrons. NH3 Lewis Structure The Lewis structure of a molecule helps understand the electron geometry, molecular geometry, polarity, and other such properties with ease. It is a pictorial representation of the arrangement of valence electrons around the individual atoms in the molecule.
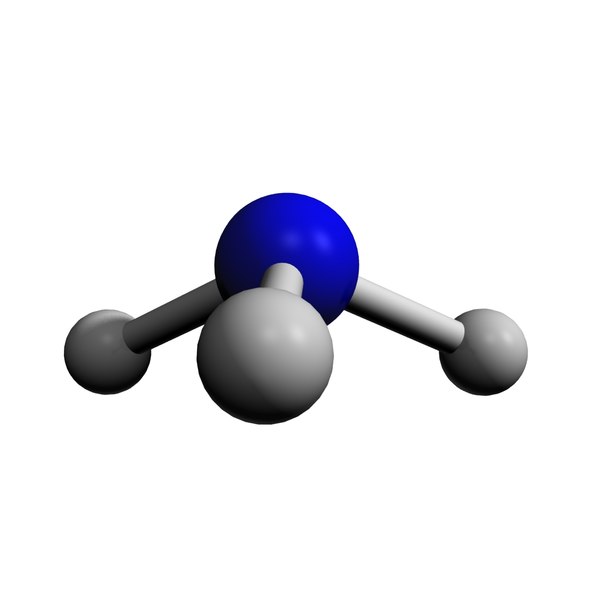
3d nh3
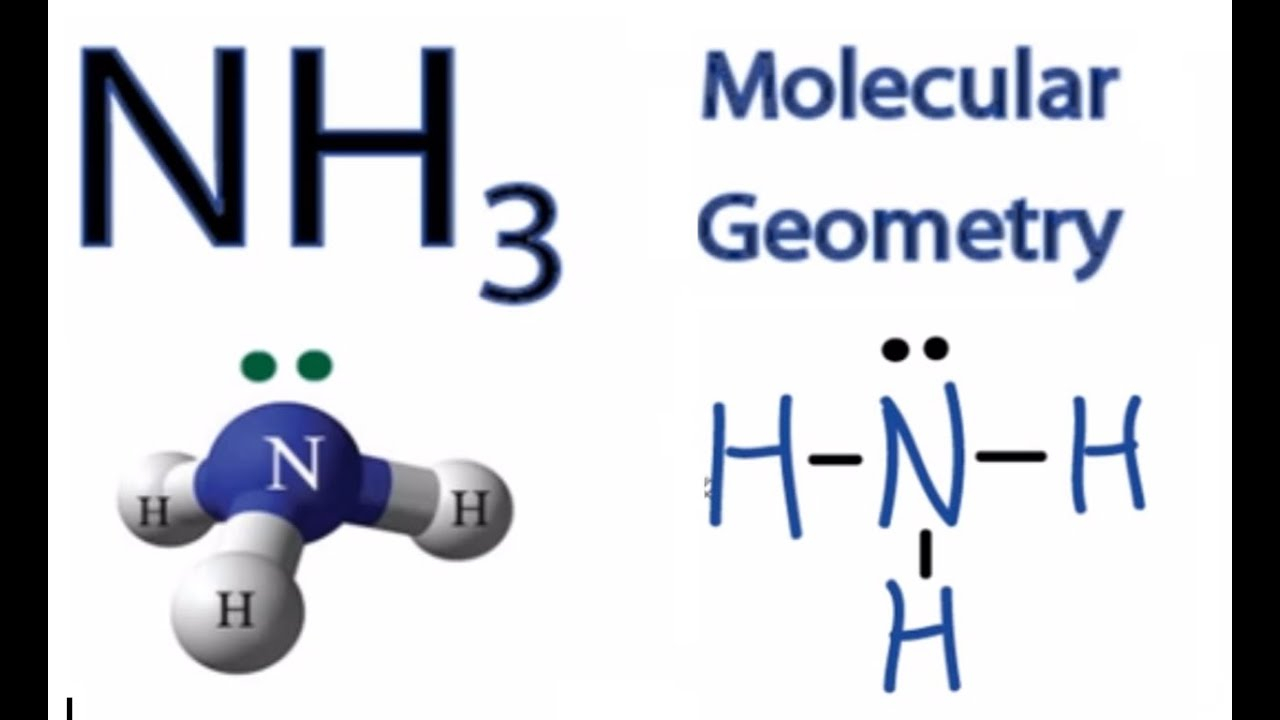
The NH3 Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the NH3 molecule. The geometry of the NH3 molecule can then be predicted using the Valence Shell Electron Pair Repulsion Theory (VSEPR Theory), which states that molecules will choose the NH3 geometrical shape in which the electrons have from one another.

NH3 Molecular Geometry Science Education and Tutorials
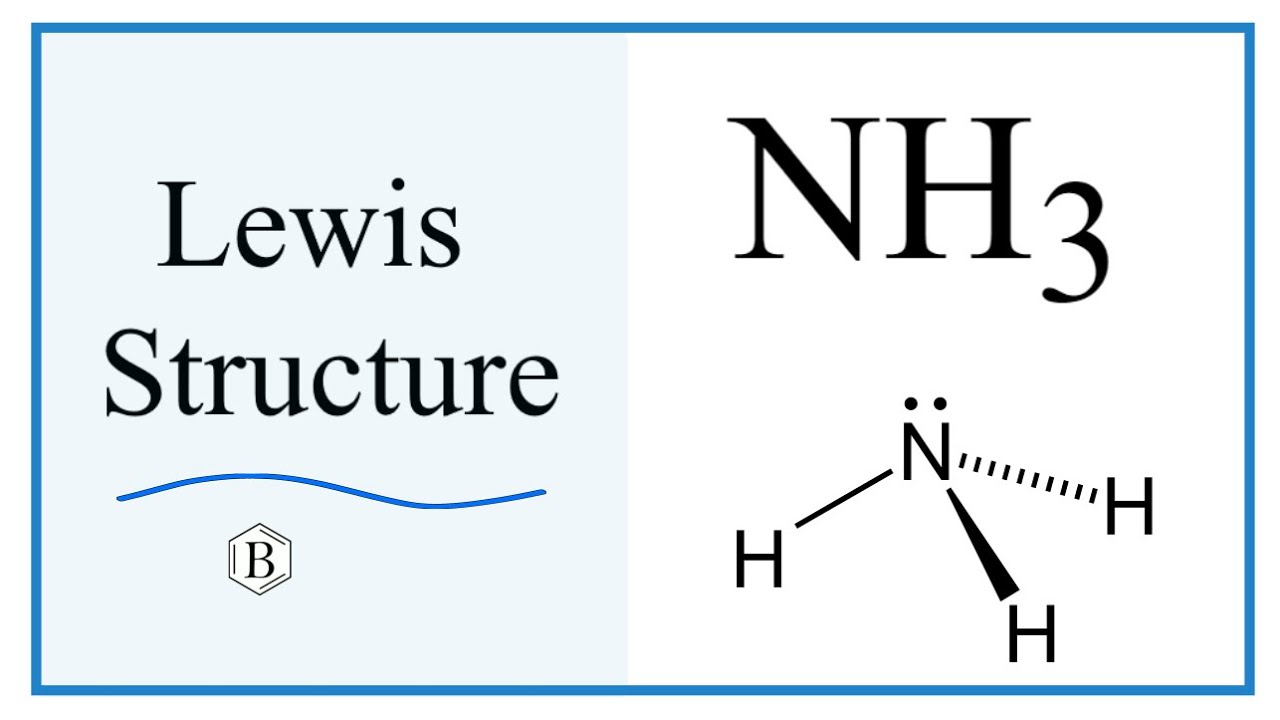
Lewis structure of NH3 (ammonia) contains three single bonds between the Nitrogen (N) atom and each Hydrogen (H) atom. The Nitrogen atom (N) is at the center and it is surrounded by 3 Hydrogen atoms (H). The Nitrogen atom has one lone pair. Let's draw and understand this lewis dot structure step by step.
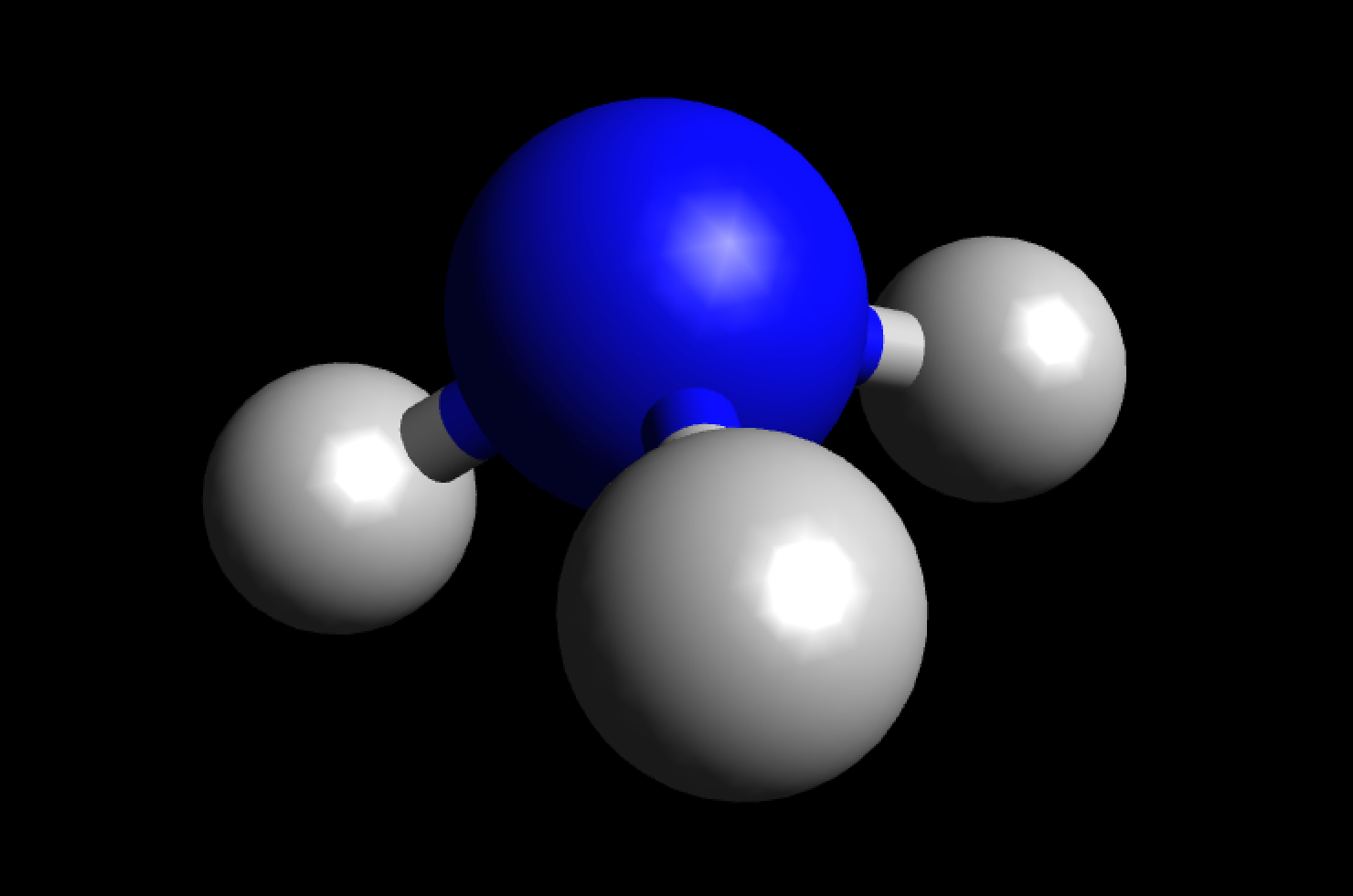
MakeTheBrainHappy Is NH3 Polar or Nonpolar?
In the NH 3 Lewis structure, there are three single bonds around the nitrogen atom, with three hydrogen atoms attached to it, and on the nitrogen atom, there is one lone pair. NH3 Lewis Structure - How to Draw the Dot Structure for NH3 (Ammonia) Watch on Contents Steps #1 Draw a rough sketch of the structure
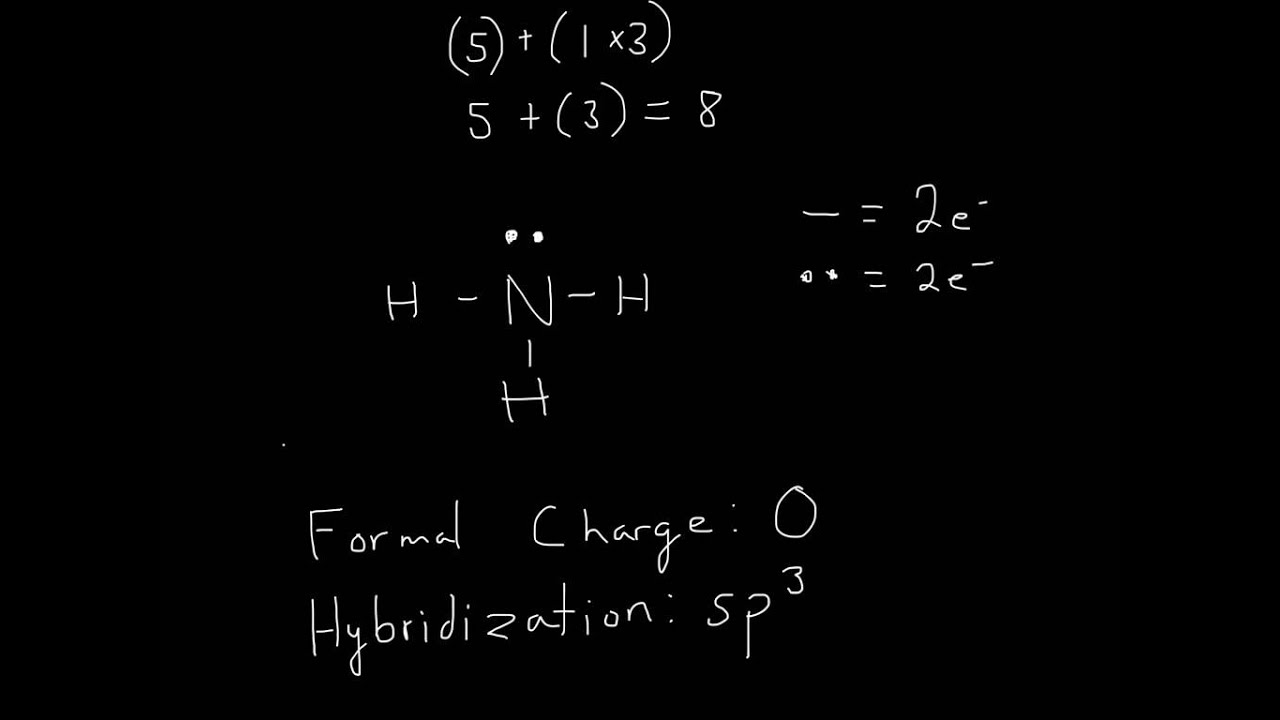
Lewis Structure of NH3 YouTube
A video explanation of how to draw the Lewis Dot Structure for Ammonia, along with information about the compound including Formal Charges, Polarity, Hybrid.

Lewis Dot Diagram Of Nh3
To begin drawing the NH3 Lewis structure, start by counting the total number of valence electrons. Valence electrons are the outermost electrons of an atom and are involved in bonding. For NH3, nitrogen (N) is in Group 5A (Group 15), so it has five valence electrons.
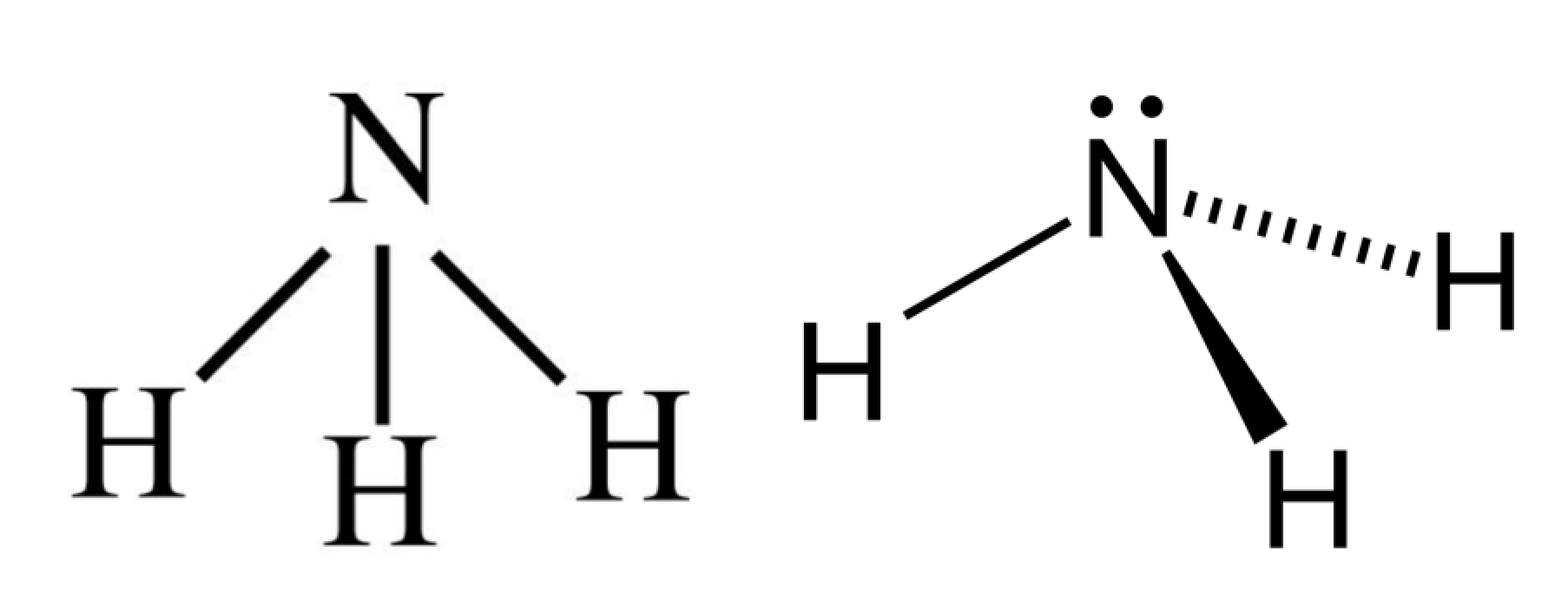
Lewis Dot Diagram For Nh3 exatin.info
NH3 Lewis structure: Steps involved in the NH3 Lewis structure: Step 1: Valance Electron Determination: Step 2: Central Metal Atom: Step 3: Connect the Atoms with a lone pair: Step 4: Distribute Lone Pairs: Step 5: Complete the Lewis Structure: Step 6: Formal Charge: Detailed applications: Synthesis/Production:

Estrutura De Lewis Nh3 AskSchool
Lewis Structure Finder. This widget gets the Lewis structure of chemical compounds. Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
Nh3 Molecule Structure
NH3 Lewis Structure: Drawings, Hybridization, Shape, Charges, Pair and Detailed Facts By Lambda Geeks NH3 Lewis structure needs to be described for illustrating the knowledge about the compound. This article would be developed with the facts delivered by the Lewis structure of NH3. The facts that would be emerged in this article are:

17 Beautiful Nh3 Lewis Structure 3d Model Viela Mockup
In the NH3 lewis dot structure, there are Four atoms present, one N and three H atoms. The valence electrons for N are 5 and for three H atoms are 3. So, the total valence electrons are 5+3 = 8. Step 2 - According to the octet rule, the electrons that will be needed for the NH3 lewis dot structure will be 8 + (3*2) = 14.
Lewis Dot Diagram For Nh3 exatin.info
Ammonia (NH 3) is a commonly tested Lewis structure due to it's widespread use in agriculture as a fertilizer. It also is a good example of a molecule with a trigonal prymidal molecular geometry. There are 8 valence electrons available for the Lewis structure for NH 3. Video: Drawing the Lewis Structure for NH3 It is helpful if you:
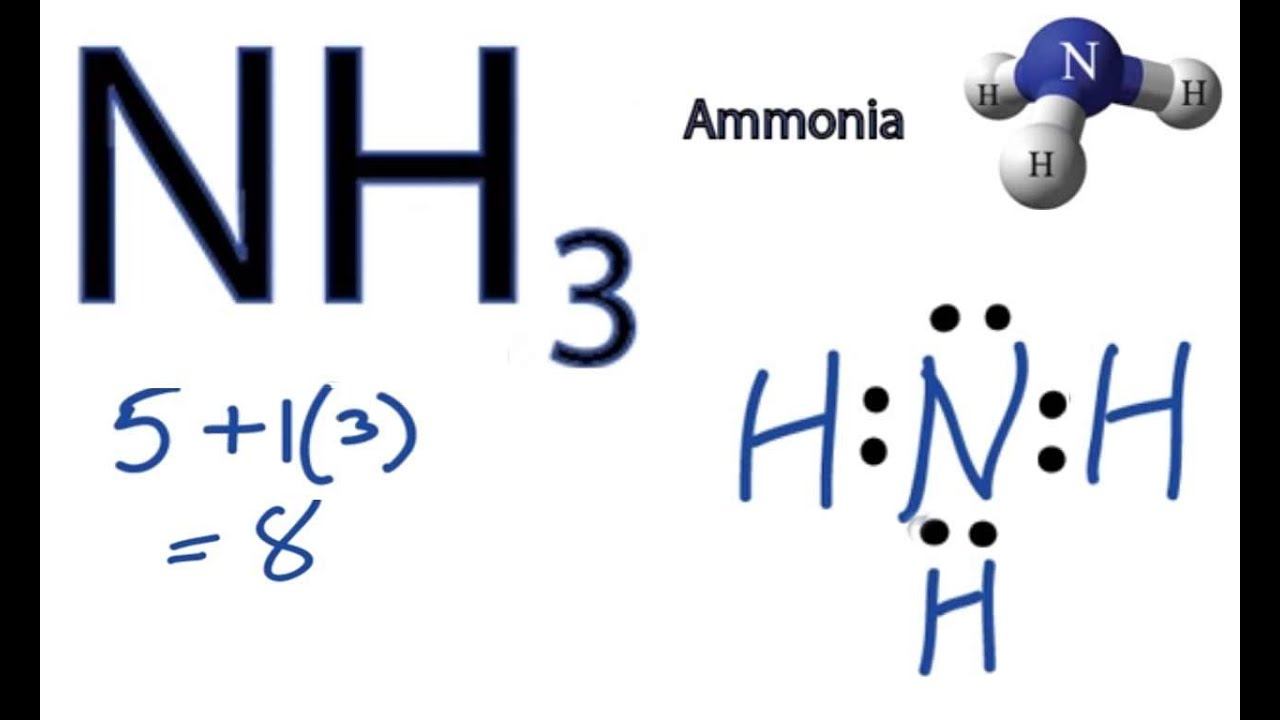
Lewis Dot Diagram For Nh3 exatin.info
There is really only one way to draw the Lewis structure for Ammonia (NH3). For resonance structures there must be a double or triple bond present, which is.
Lewis Dot Diagram For Nh3 exatin.info
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

Estructura De Lewis Del Amoníaco Nh3 Explicacion Facil Mobile Legends
A step-by-step explanation of how to draw the NH3 Lewis Dot Structure (Ammonia).For the NH3 structure use the periodic table to find the total number of vale.

electron dot structure of NH3 Science Carbon and its Compounds
1: Structure and Bonding 1.3: Lewis Structures Expand/collapse global location 1.3: Lewis Structures Page ID Using Lewis Dot Symbols to Describe Covalent Bonding
